| Structure | Name/CAS No. | Articles |
|---|---|---|
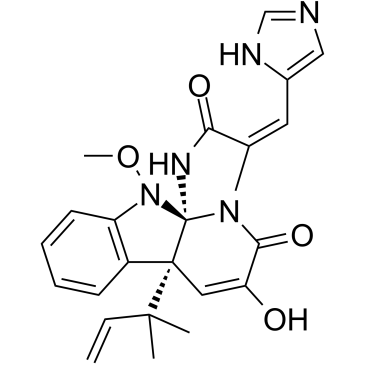 |
Meleagrin
CAS:71751-77-4 |
S Odani, T Koide, T Ono, Y Takahashi, J Suzuki
Index: J. Biochem. 105(4) , 660-3, (1989)
Full Text: HTML
A low-molecular-mass protein, tentatively named meleagrin, was isolated from a commercial preparation of turkey (Meleagris gallopavo) ovomucoid. This 40-amino acid protein contains 3 disulfide bonds and high concentrations of aromatic residues (2 tryptophans and 3 tyrosines). It lacks threonine, methionine, phenylalanine, and arginine residues. The complete amino acid sequence was determined to be the following: less than Glu-Val-Leu-Lys-Tyr-Cys-Pro-Lys-Ile-Gly-Tyr-Cys-Ser-Ser-Lys-Cys-Ser-Lys- Ala- Glu-Val-Trp-Ala-Tyr-Ser-Pro-Asp-Cys-Lys-Val-His-Cys-Cys-Val-Pro-Ala-Asn- Gln-Lys - Trp. One of the three disulfide bonds exists between Cys12 and Cys28, and the two others links Cys32-Cys33 with Cys6 and Cys16. The amino acid sequence of meleagrin shows a strong homology to a similar basic protein, cygnin (Simpson, G.R. & Morgan, F.J. [1983] Int. J. Pep. Protein Res. 22, 476-481), of a rather distantly related aves, black swan (Cygnus atratus), suggesting some vital role of this protein in avian eggs. Similarity to a part (exon 9) of transferrins was also recognized.
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
 |
Meleagrin
CAS:71751-77-4 |
C23H23N5O4 |
|
[The biosynthesis of low-molecular-weight nitrogen-containin...
2002-01-01 [Mikrobiologiia 71(6) , 773-7, (2002)] |
|
A single cluster of coregulated genes encodes the biosynthes...
2011-11-23 [Chem. Biol. 18(11) , 1499-512, (2011)] |
|
Roquefortine/oxaline biosynthesis pathway metabolites in Pen...
2005-10-01 [J. Chem. Ecol. 31(10) , 2373-90, (2005)] |
Home | MSDS/SDS Database Search | Journals | Product Classification | Biologically Active Compounds | Selling Leads | About Us | Disclaimer
Copyright © 2026 ChemSrc All Rights Reserved