Preparation and pharmacological evaluation of novel glycoprotein (Gp) IIb/IIIa antagonists. 1. The selection of naphthalene derivatives.
S Ono, Y Inoue, T Yoshida, A Ashimori, K Kosaka, T Imada, C Fukaya, N Nakamura
Index: Chem. Pharm. Bull. 47(12) , 1685-93, (1999)
Full Text: HTML
Abstract
The synthesis and design using molecular modeling techniques for non-peptide, low molecular weight novel fibrinogen receptor (glycoprotein IIb/IIIa: Gp IIb/IIIa) antagonists, is reported. We used a highly potent serine protease inhibitor, Nafamostat, having an amidinonaphthyl unit as the starting compound. The compounds 4-(6-amidino-2-naphthylaminocarbonyl)phenoxyacetic acid (5a) and 4-(6-amidino-2-naphthalenecarboxamido)phenoxyacetic acid (5b) inhibited adenosin-5'-diphospate (ADP)-induced aggregation of human platelet-rich plasma (PRP) with IC50 values of 0.05 and 0.07 microM, respectively, and had lost their ability to inhibit a variety of serine proteases, including thrombin, factor Xa, plasmin and trypsin.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
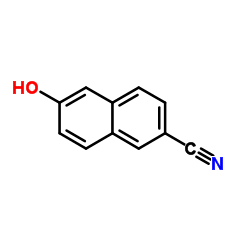 |
6-Hydroxy-2-naphthonitrile
CAS:52927-22-7 |
C11H7NO |
|
Synthesis and structure-activity study of protease inhibitor...
1993-01-01 [Chem. Pharm. Bull. 41(1) , 117-25, (1993)] |
|
Exploration of the active site of β4GalT7: modifications of ...
2015-03-21 [Org. Biomol. Chem. 13(11) , 3351-62, (2015)] |
|
Synthesis and anti-HIV activity of 2-naphthyl substituted DA...
2010-07-01 [Bioorg. Med. Chem. 18(13) , 4601-5, (2010)] |
|
Minor groove binding compounds that jump a gc base pair and ...
2009-02-24 [Biochemistry 48(7) , 1573-83, (2009)] |
|
Doping of Polyaniline with 6-Cyano-2-naphthol. Das D, et al.
[J. Phys. Chem. B 118(45) , 12993-13001, (2014)] |