Chirality
2005-10-01
Chiral aminonaphthol-catalyzed enantioselective carbonyl addition of diethylzinc to aromatic aldehydes high-throughput screened by CD-HPLC analysis.
Ling Xu, Xiumin Shen, Cong Zhang, Koichi Mikami
Index: Chirality 17(8) , 476-80, (2005)
Full Text: HTML
Abstract
Optically active aminonaphthols derivatives are obtained by condensation of 2-naphthol, substituted benzaldehyde, and (S)-methylbenzylamine under mild conditions, without side products. Their absolute configurations are determined by X-ray crystallographic analysis. The addition of diethylzinc to aromatic aldehydes is considerably accelerated by the presence of a catalytic amount of crystalline to give, after hydrolysis, the corresponding 1-phenylpropanol in good enantiomeric purity, as determined by CD-HPLC analysis as HTPS (high-throughput screening).
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
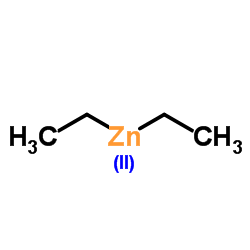 |
Diethylzinc
CAS:557-20-0 |
C4H10Zn |
Related Articles:
More...
|
Copper-catalyzed asymmetric conjugate addition of diethylzin...
2006-11-09 [Org. Lett. 8 , 5405, (2006)] |
|
(R)-(+)-Binol-functionalized mesoporous organosilica as a hi...
2010-05-03 [Chem. Asian J. 5(5) , 1232-9, (2010)] |
|
Highly enantioselective addition of terminal alkynes to alde...
2009-02-01 [Chirality 21(2) , 316-23, (2009)] |
|
Enantioselective Reformatsky reaction of ethyl iododifluoroa...
2012-04-28 [Org. Biomol. Chem. 10(16) , 3332-42, (2012)] |
|
Efficient chirality switching in the addition of diethylzinc...
2007-01-01 [Angew. Chem. Int. Ed. Engl. 46(47) , 9002-5, (2007)] |