| Structure | Name/CAS No. | Articles |
|---|---|---|
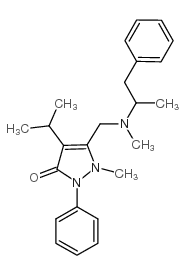 |
Famprofazone
CAS:22881-35-2 |
|
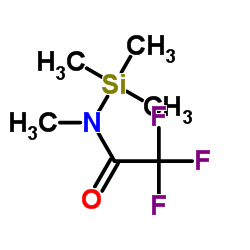 |
MSTFA
CAS:24589-78-4 |
|
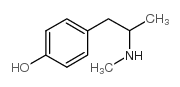 |
Pholedrine
CAS:370-14-9 |