Carbohydrate Research
2010-09-23
Activation of glycosyl trichloroacetimidates with perchloric acid on silica (HClO(4)-SiO(2)) provides enhanced alpha-selectivity.
Olaf R Ludek, Wenlu Gu, Jeffrey C Gildersleeve
Index: Carbohydr. Res. 345(14) , 2074-8, (2010)
Full Text: HTML
Abstract
Obtaining high stereoselectivity in glycosylation reactions is often challenging in the absence of neighboring group participation. In this study, we demonstrate that activation of glycosyl trichloroacetimidate donors with immobilized perchloric acid on silica (HClO(4)-SiO(2)) provides higher alpha-selectivity than trimethylsilyl triflate (TMSOTf) for reactions that do not involve neighboring group participation.Published by Elsevier Ltd.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
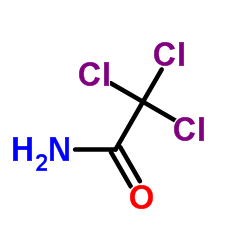 |
2,2,2-Trichloroacetamide
CAS:594-65-0 |
C2H2Cl3NO |
Related Articles:
More...
|
An improved synthesis of (-)-5,11-dideoxytetrodotoxin.
2013-02-15 [J. Org. Chem. 78(4) , 1699-705, (2013)] |
|
Synthetic studies toward the anthrax tetrasaccharide: altern...
2012-07-15 [Carbohydr. Res. 356 , 115-31, (2012)] |
|
Stereoselective rearrangement of trichloroacetimidates: appl...
2014-01-01 [Org. Lett. 11(11) , 2433-6, (2009)] |
|
Rhodium-catalyzed regio- and enantioselective amination of r...
2012-12-07 [Chem. Commun. (Camb.) 48(94) , 11531-3, (2012)] |
|
Influence of the solvent in low temperature glycosylations w...
2011-09-06 [Carbohydr. Res. 346(12) , 1495-502, (2011)] |