| Structure | Name/CAS No. | Articles |
|---|---|---|
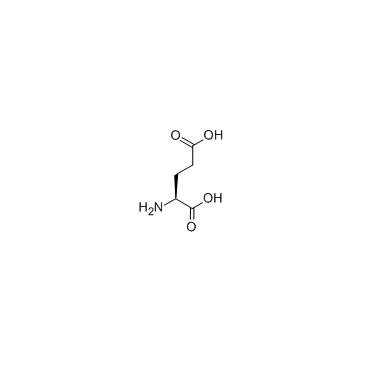 |
L-glutamic acid
CAS:56-86-0 |
|
 |
L-AP4
CAS:23052-81-5 |