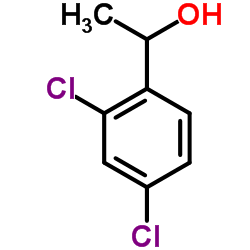
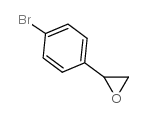
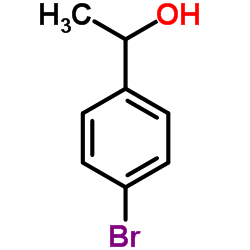
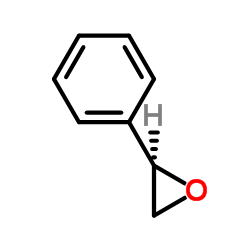
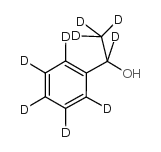
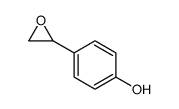
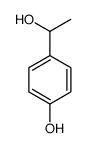
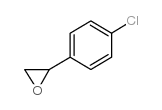
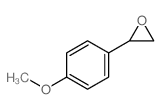
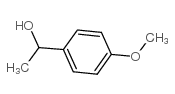
|
~85% |
|
~86% |
|
~87% |
|
~84% |
|
~85% |
|
~88% |
|
~79% |