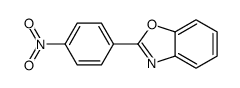
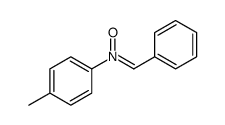
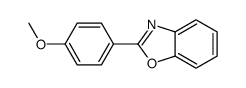
|
~51% |
|
~70% |
|
~80% |
|
~70% |
|
~60% |

![2-[(4-methoxyphenyl)methylideneamino]-4-methylphenol Structure](https://image.chemsrc.com/caspic/436/19430-23-0.png)
![2-(4-METHOXYPHENYL)-5-METHYLBENZO[D]OXAZOLE Structure](https://image.chemsrc.com/caspic/137/35876-70-1.png)