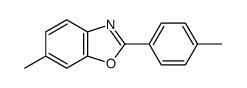
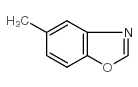
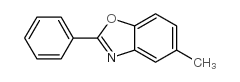
|
~90% |
|
~91% |
|
~41% |
|
~75% |
|
~71% |
|
~63% |
|
~72% |
|
~53% |
|
~73% |
|
~56% |
|
~92% |
|
~80% |
|
~86% |
|
~80% |
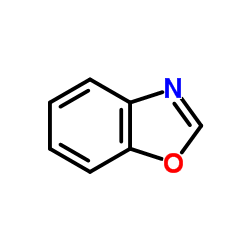
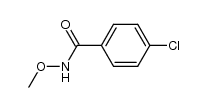


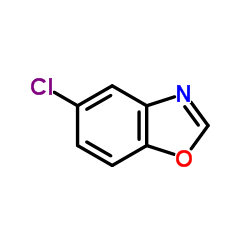
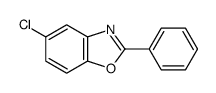
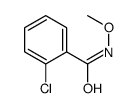
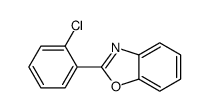
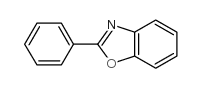
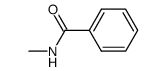
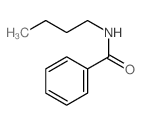
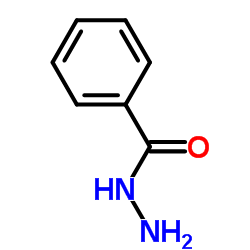
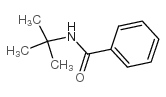
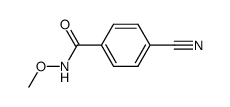
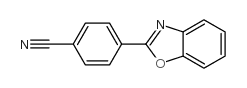
![Benzo[d]thiazole Structure](https://image.chemsrc.com/caspic/318/95-16-9.png)
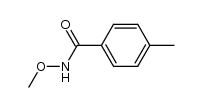

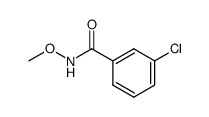
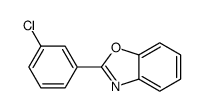

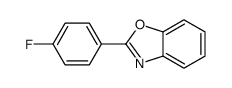
![6-METHYLBENZO[D]OXAZOLE Structure](https://image.chemsrc.com/caspic/271/10531-80-3.png)