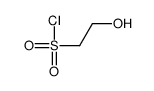
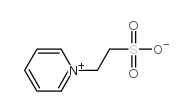
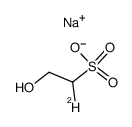
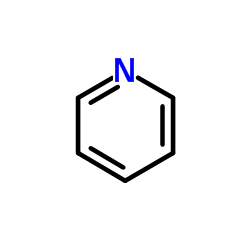
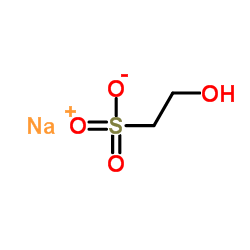
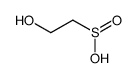
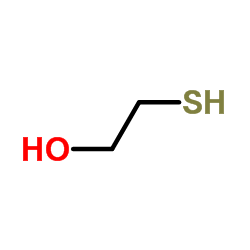
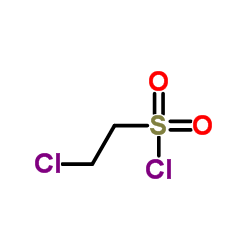
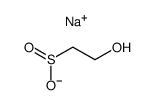
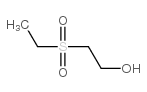
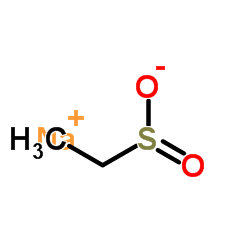
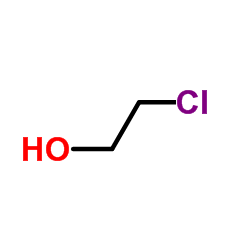
|
~% |
|
~55%
Detail
|
|
~74% |
|
~30% |
|
~% |
|
~5% |