Determination of serotonin, noradrenaline, dopamine and their metabolites in rat brain extracts and microdialysis samples by column liquid chromatography with fluorescence detection following derivatization with benzylamine and 1,2-diphenylethylenediamine.
Takashi Yoshitake, Jan Kehr, Shimako Yoshitake, Kaoru Fujino, Hitoshi Nohta, Masatoshi Yamaguchi
Index: J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 807(2) , 177-83, (2004)
Full Text: HTML
Abstract
A highly selective and sensitive column liquid chromatographic method for fluorescence determination of serotonin (5-HT), dopamine (DA), noradrenaline (NA) and their related metabolites 5-hydroxyindole-3-acetic acid (5-HIAA) and 3,4-dihydroxyphenylacetic acid (DOPAC) following derivatization with benzylamine and 1,2-diphenylethylenediamine (DPE) is described. The monoamines and the metabolites (20 microl samples) were derivatized in a two-step reaction, initiated with 20 microl of 0.3M benzylamine in 0.3M 3-cyclohexylaminopropanesulfonic acid (CAPS) buffer (pH 10.0), (for 5-HT, 5-HIAA, 2 min, 24 degrees C) and followed by 20 microl of 0.1M DPE in 0.3M glycine buffer (pH 10.0), (for DA, NA, DOPAC, 20 min, 50 degrees C). Both reagents contained 0.02 M potassium hexacyanoferrate(III) and 50% (v/v) methanol. The resulting highly fluorescent and stable benzoxazole derivatives were isocratically separated on a reversed-phase column (150 mm x 1.5 mm i.d., packed with C18 silica, 5 microm) within 45 min. Using fluorescence detection at ex. and em. wavelengths of 345 and 480 nm, respectively, the detection limit (signal-to-noise ratio of 3) for 5-HT, DA, NA, 5-HIAA, L-DOPA and DOPAC ranged between 0.08 and 5.65 fmol per 20-microl injection (12-847.5 pM in standard solution). The concentrations of monoamines (expressed in microg/g wet weight, mean +/- S.E.M., n=5) in tissue extracts from the rat striatum were: 0.45+/-0.05 (5-HT), 4.27+/-0.08 (DA), 0.27+/-0.04 (NA), 0.55+/-0.06 (5-HIAA), 1.26+/-0.16 (L-DOPA) and 1.62+/-0.11 (DOPAC). Microdialysis samples were collected in 20 min intervals from the probes implanted in the striatum of awake rats. The basal monoamine levels (in fmol/20 microl, mean +/- S.E.M., n=5) in the dialysates were: 4.0+/-0.7 (5-HT), 78.4+/-9.1 (DA), 6.4+/-0.8 (NA), 785.5+/-64.5 (5-HIAA) and 5504.5+/-136.5 (DOPAC). It is concluded that the new fluorescence derivatization protocol provides an excellent means for simultaneous determination of all three monoamines both in the complex samples (e.g. brain homogenates) and also at trace levels, such as those found in the microdialysis samples.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
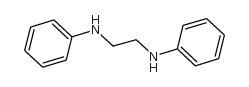 |
1,2-Ethanediamine,N1,N2-diphenyl
CAS:150-61-8 |
C14H16N2 |
|
Role of dermal exposure in systemic intake of methylenediphe...
2015-02-03 [Toxicol. Lett. 232(3) , 595-600, (2015)] |
|
Reversible and irreversible interactions of a cisplatin anal...
1994-01-01 [Drug Metab. Dispos. 22(3) , 419-27, (1994)] |
|
Preparation and enantioseparation of a mixed selector chiral...
2010-06-01 [Chirality 22(6) , 604-11, (2010)] |
|
Synthesis of dendritic stationary phases with surface-bonded...
2008-07-01 [Chirality 20(7) , 846-55, (2008)] |
|
Synthesis of polymer-type chiral stationary phases and their...
2007-02-01 [Chirality 19(2) , 129-40, (2007)] |