Synthesis of dendritic stationary phases with surface-bonded L-phenylalanine derivate as chiral selector and their evaluation in HPLC resolution of racemic compounds.
Chuan-Qi Yin, Bao-Jiang He, Shao-Hua Huang, Jun-Yi Zhang, Zheng-Wu Bai, Zao-Ying Li
Index: Chirality 20(7) , 846-55, (2008)
Full Text: HTML
Abstract
Four dendrimers were synthesized on aminopropyl-modified silica gel using methyl acrylate and ethylene diamine as building blocks by divergent method. Four generations of chiral stationary phases (CSPs) were prepared by coupling of L-2-(p-toluenesulfonamido)-3-phenylpropionyl chloride to corresponding dendrimers. The derivatives prepared on silica gel were characterized by FT-IR, (1)H NMR, and elemental analysis. The selector loadings of these four generations of CSPs generally showed a decrease tendency with the increase of generation numbers of dendrimers. The enantioseparation properties of these CSPs were preliminarily investigated by high-performance liquid chromatography. The CSP derived from the three-generation dendrimer exhibited the best enantioseparation capability. Effects of the mobile phase composition and molecular structures of racemic mixtures on enantioseparation were further studied.Copyright 2008 Wiley-Liss, Inc.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
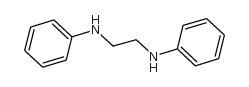 |
1,2-Ethanediamine,N1,N2-diphenyl
CAS:150-61-8 |
C14H16N2 |
|
Role of dermal exposure in systemic intake of methylenediphe...
2015-02-03 [Toxicol. Lett. 232(3) , 595-600, (2015)] |
|
Reversible and irreversible interactions of a cisplatin anal...
1994-01-01 [Drug Metab. Dispos. 22(3) , 419-27, (1994)] |
|
Preparation and enantioseparation of a mixed selector chiral...
2010-06-01 [Chirality 22(6) , 604-11, (2010)] |
|
Synthesis of polymer-type chiral stationary phases and their...
2007-02-01 [Chirality 19(2) , 129-40, (2007)] |
|
Spectrofluorometric determination of catechins with 1,2-diph...
2002-08-01 [Anal. Sci. 18(8) , 951-3, (2002)] |