Selenide retention by mackinawite.
N Finck, K Dardenne, D Bosbach, H Geckeis
Index: Environ. Sci. Technol. 46(18) , 10004-11, (2012)
Full Text: HTML
Abstract
The isotope (79)Se may be of great concern with regard to the safe disposal of nuclear wastes in deep geological repositories due to its long half-life and potential mobility in the geosphere. The Se mobility is controlled by the oxidation state: the oxidized species (Se(IV)) and (Se(VI)) are highly mobile, whereas the reduced species (Se(0) and Se(-II)) form low soluble solids. The mobility of this trace pollutant can be greatly reduced by interacting with the various barriers of the repository. Numerous studies report on the oxidized species retention by mineral phases, but only very scarce studies report on the selenide (Se(-II)) retention. In the present study, the selenide retention by coprecipitation with and by adsorption on mackinawite (FeS) was investigated. XRD and SEM analyses of the samples reveal no significant influence of Se on the mackinawite precipitate morphology and structure. Samples from coprecipitation and from adsorption are characterized at the molecular scale by a multi-edge X-ray absorption spectroscopy (XAS) investigation. In the coprecipitation experiment, all elements (S, Fe, and Se) are in a low ionic oxidation state and the EXAFS data strongly point to selenium located in a mackinawite-like sulfide environment. By contacting selenide ions with FeS in suspension, part of Se is located in an environment similar to that found in the coprecipitation experiment. The explanation is a dynamical dissolution-recrystallization mechanism of the highly reactive mackinawite. This is the first experimental study to report on selenide incorporation in iron monosulfide by a multi-edge XAS approach.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
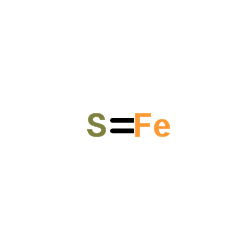 |
Ferrous sulfide
CAS:1317-37-9 |
FeS |
|
Enhanced reductive dechlorination of tetrachloroethene durin...
2012-10-15 [J. Hazard. Mater. 235-236 , 359-66, (2012)] |
|
Black stain - a review.
2011-01-01 [Oral Health Prev. Dent. 9(1) , 37-45, (2011)] |
|
The mechanism of the reduction of [AnO2]2+ (An = U, Np, Pu) ...
2011-11-14 [Dalton Trans. 40(42) , 11156-63, (2011)] |
|
FeS/S/FeS(2) redox system and its oxidoreductase-like chemis...
2011-06-01 [Astrobiology 11(5) , 471-6, (2011)] |
|
Removal of highly elevated nitrate from drinking water by pH...
2011-11-01 [Bioresour. Technol. 102(21) , 10154-7, (2011)] |