Tumor reactive cis-aconitylated monoclonal antibodies coupled to daunorubicin through a peptide spacer are unable to kill tumor cells.
K L Law, K E Studtmann, R E Carlson, T A Swanson, A W Buirge, A Ahmad
Index: Anticancer Res. 10(3) , 845-52, (1990)
Full Text: HTML
Abstract
Antibody-drug conjugates containing a linkage susceptible to lysosomal hydrolases were constructed by coupling peptide-daunorubicin (DNR) derivatives to MAb. Using a modification in the method of Trouet et al, peptide derivatives of DNR containing the sequences Ala-Leu and Ala-Leu-Ala-Leu linked to drug via their carboxy terminus were prepared. Cleavage of these derivatives by lysosomal enzymes resulting in the release of free DNR was demonstrated. Human antitumor MAb were derivatized with either succinic anhydride or cis-aconitic anhydride to introduce spacer arms for coupling. Binding studies showed that MAb with a decrease of 12-20 amino groups retained greater than 70% of their immunoreactivity, a level deemed acceptable for constructing conjugates. Derivatized and native MAb were conjugated to peptide-DNR via a carbodiimide mediated reaction. None of the conjugates displayed cytotoxicity toward target tumor cell lines in vitro.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
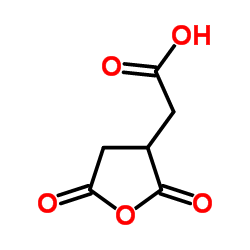 |
(2,5-Dioxotetrahydro-3-furanyl)acetic acid
CAS:6318-55-4 |
C6H4O5 |
|
Enhanced shRNA delivery and ABCG2 silencing by charge-revers...
2015-02-25 [Small 11(8) , 952-62, (2015)] |
|
Development of biodegradable polymeric implants of RGD-modif...
2015-05-01 [Drug Deliv. 22 , 389-99, (2015)] |
|
Acid-responsive PEGylated doxorubicin prodrug nanoparticles ...
2015-12-01 [Colloids Surf. B Biointerfaces 136 , 365-74, (2015)] |
|
Cerebellar Purkinje cells incorporate immunoglobulins and im...
2009-01-01 [J. Neuroinflammation 6 , 31, (2009)] |
|
Lectin-mediated drug targeting: preparation, binding charact...
1998-07-01 [Pharm. Res. 15(7) , 1031-7, (1998)] |