| Structure | Name/CAS No. | Articles |
|---|---|---|
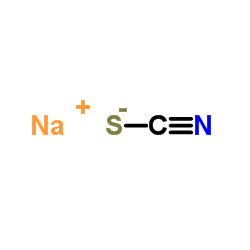 |
Sodium thiocyanate
CAS:540-72-7 |
|
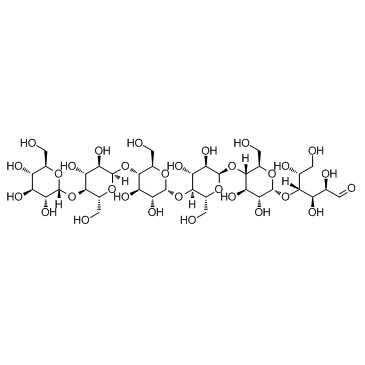 |
Maltohexaose
CAS:34620-77-4 |