| Structure | Name/CAS No. | Articles |
|---|---|---|
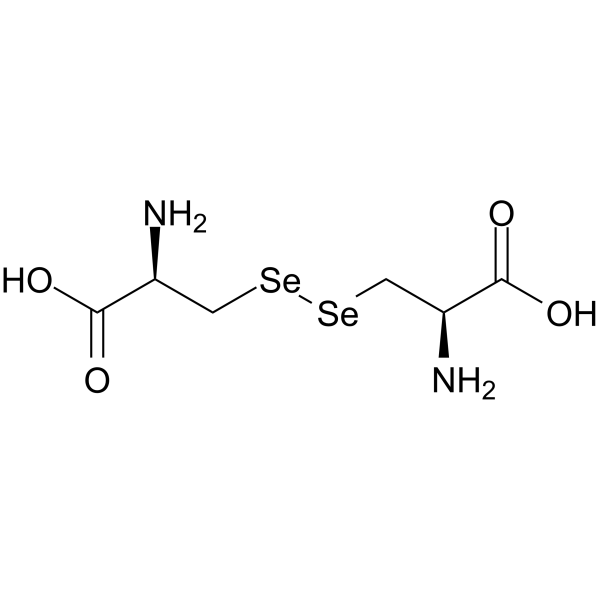 |
selenocystine
CAS:29621-88-3 |
|
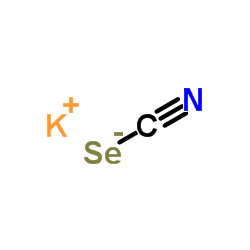 |
Potassium selenocyanate
CAS:3425-46-5 |
|
 |
Seleno-DL-cystine
CAS:2897-21-4 |