In situ gelling hexagonal phases for sustained release of an anti-addiction drug.
Jessica Phelps, M Vitória L B Bentley, Luciana B Lopes
Index: Colloids Surf. B Biointerfaces 87(2) , 391-8, (2011)
Full Text: HTML
Abstract
In this study, fluid precursor formulations for subcutaneous injection and in situ formation of hexagonal phase gels upon water absorption were developed as a strategy to sustain the release of naltrexone, a drug used for treatment of drug addiction. Precursor formulations were obtained by combining BRIJ 97 with propylene glycol (PG, 5-70%, w/w). To study the phase behavior of these formulations, water was added at 10-90% (w/w), and the resulting systems were characterized by polarized light microscopy. Two precursor formulations containing BRIJ:PG at 95:5 (w/w, referred to as BRIJ-95) and at 80:20 (w/w, referred to as BRIJ-80) were chosen. Naltrexone was dissolved at 1% or suspended at 5% (w/w). Precursor formulations were transformed into hexagonal phases when water content exceeded 20%. Water uptake followed second-order kinetics, and after 2-4h all precursor formulations were transformed into hexagonal phases. Drug release was prolonged by the precursor formulations (compared to a drug solution in PBS), and followed pseudo-first order kinetics regardless of naltrexone concentration. The release from BRIJ-80 was significantly higher than that from BRIJ-95 after 48 h. The relative safety of the precursor formulations was assessed in cultured fibroblasts. Even though BRIJ-95 was more cytotoxic than BRIJ-80, both precursor formulations were significantly less cytotoxic than sodium lauryl sulfate (considered moderate-to-severe irritant) at the same concentration (up to 50 μg/mL). These results suggest the potential of BRIJ-based precursor formulations for sustained naltrexone release.Copyright © 2011 Elsevier B.V. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
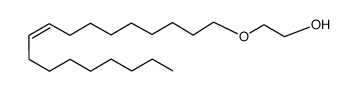 |
Genapol O
CAS:9004-98-2 |
C20H40O2 |
|
Antimicrobial Nanoemulsion Formulation with Improved Penetra...
2015-01-01 [PLoS ONE 10 , e0133826, (2015)] |
|
TLR adaptor MyD88 is essential for pathogen control during o...
2008-09-01 [J. Immunol. 181(5) , 3464-73, (2008)] |
|
Key structure of brij for overcoming multidrug resistance in...
2013-02-11 [Biomacromolecules 14(2) , 424-30, (2013)] |
|
Solubilisation capacity of Brij surfactants.
2012-10-15 [Int. J. Pharm. 436(1-2) , 631-5, (2012)] |
|
Enhanced percutaneous permeability of diclofenac using a new...
2008-04-01 [Drug Dev. Ind. Pharm. 34(4) , 403-12, (2008)] |