| Structure | Name/CAS No. | Articles |
|---|---|---|
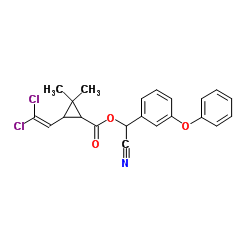 |
Cypermethrin
CAS:52315-07-8 |
|
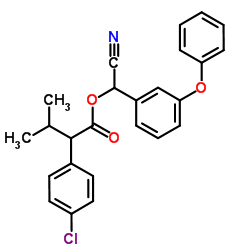 |
fenvalerate
CAS:51630-58-1 |
|
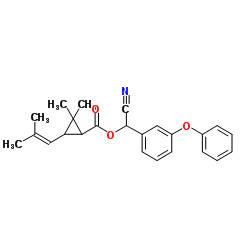 |
Cyphenothrin
CAS:39515-40-7 |
|
 |
Esfenvalerate
CAS:66230-04-4 |
|
![fenpropathrin [ANSI] Structure](https://image.chemsrc.com/caspic/322/39515-41-8.png) |
fenpropathrin [ANSI]
CAS:39515-41-8 |
|
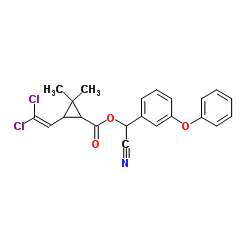 |
α-cypermethrin
CAS:67375-30-8 |