| Structure | Name/CAS No. | Articles |
|---|---|---|
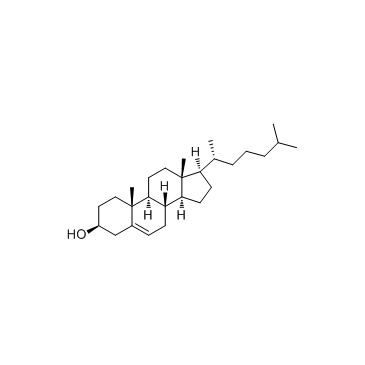 |
cholesterol
CAS:57-88-5 |
|
 |
3,4-Dihydro-2H-pyran
CAS:110-87-2 |
|
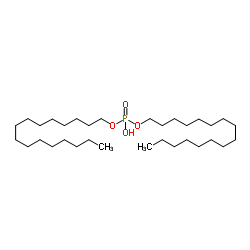 |
Dicetylphosphate
CAS:2197-63-9 |
|
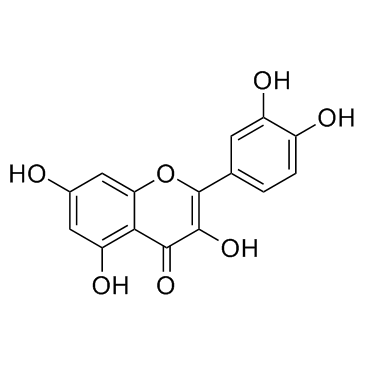 |
Quercetin
CAS:117-39-5 |