| Structure | Name/CAS No. | Articles |
|---|---|---|
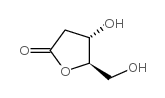 |
(4S,5R)-4-Hydroxy-5-(hydroxymethyl)dihydrofuran-2(3H)-one
CAS:34371-14-7 |
|
 |
Deoxyribonucleic acids, thymus gland, sodium salts
CAS:73049-39-5 |