Histone-catalyzed cleavage of nucleosomal DNA containing 2-deoxyribonolactone.
Chuanzheng Zhou, Marc M Greenberg
Index: J. Am. Chem. Soc. 134(19) , 8090-3, (2012)
Full Text: HTML
Abstract
Oxidized abasic sites such as 2-deoxyribonolactone (L) are produced in DNA by a variety of oxidizing agents, including potent cytotoxic antitumor natural products. 2-Deoxyribonolactone is labile under alkaline conditions, but its half-life in free DNA at pH 7.5 is approximately 1 week. Independent generation of L at defined positions within nucleosomes reveals that the histone proteins catalyze strand scission and increase the rate between 11- and ∼43-fold. Mechanistic studies indicate that DNA-protein cross-links are not intermediates en route to strand scission and that C2 deprotonation is the rate-determining step. The use of mutant histone H4 proteins demonstrates that the lysine-rich tail that is often post-translationally modified in cells contributes to the cleavage of L but is not the sole source of the enhanced cleavage rates. Consideration of DNA repair in cells suggests that L formation in nucleosomal DNA as part of bistranded lesions by antitumor antibiotics results in de facto double strand breaks, the most deleterious form of DNA damage.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
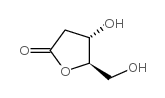 |
(4S,5R)-4-Hydroxy-5-(hydroxymethyl)dihydrofuran-2(3H)-one
CAS:34371-14-7 |
C5H8O4 |
|
Reduced repair capacity of a DNA clustered damage site compr...
2014-04-01 [Mutat. Res. Fundam. Mol. Mech. Mutagen. 762 , 32-9, (2014)] |
|
Analysis of base excision DNA repair of the oxidative lesion...
2006-01-01 [Meth. Enzymol. 408 , 48-64, (2006)] |
|
Mutagenic effects of abasic and oxidized abasic lesions in S...
2005-01-01 [Nucleic Acids Res. 33(19) , 6196-202, (2005)] |
|
Use of fluorescence sensors to determine that 2-deoxyribonol...
2007-01-01 [Angew. Chem. Int. Ed. Engl. 46(4) , 561-4, (2007)] |
|
2-Deoxyribonolactone lesions in X-ray-irradiated DNA: quanti...
2005-09-26 [Angew. Chem. Int. Ed. Engl. 44(38) , 6210-3, (2005)] |