4,6-O-[1-cyano-2-(2-iodophenyl)ethylidene] acetals. improved second-generation acetals for the stereoselective formation of beta-D-mannopyranosides and regioselective reductive radical fragmentation to beta-D-rhamnopyranosides. scope and limitations.
David Crich, Albert A Bowers
Index: J. Org. Chem. 71(9) , 3452-63, (2006)
Full Text: HTML
Abstract
The [1-cyano-2-(2-iodophenyl)]ethylidene group is introduced as an acetal-protecting group for carbohydrate thioglycoside donors. The group is easily introduced under mild conditions, over short reaction times, and in the presence of a wide variety of other protecting groups by the reaction of the 4,6-diol with triethyl (2-iodophenyl)orthoacetate and camphorsulfonic acid, followed by trimethylsilyl cyanide and boron trifluoride etherate. The new protecting group conveys strong beta-selectivity with thiomannoside donors and undergoes a tin-mediated radical fragmentation to provide high yields of the synthetically challenging beta-rhamnopyranosides. The method is also applicable to the glucopyranosides when high alpha-selectivity is observed in the coupling reaction and alpha-quinovosides are formed selectively in the radical fragmentation step. In the galactopyranoside series, beta-glycosides are formed selectively on coupling to donors protected by the new system, but the radical fragmentation is unselective and gives mixtures of the 4- and 6-deoxy products. Variable-temperature NMR studies for the glycosylation step, which helped define an optimal protocol, are described.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
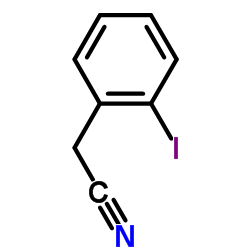 |
(2-Iodophenyl)acetonitrile
CAS:40400-15-5 |
C8H6IN |
|
Palladium(II)-Catalyzed Annulation of Alkynes with 2-(Cyanom...
2016-03-04 [J. Org. Chem. 81(5) , 1733-45, (2016)] |
|
Palladium-catalyzed carboxamidation reaction and aldol conde...
[Org. Lett. 10(21) , 4987-4990, (2008)] |
|
Palladium-catalyzed borylation of ortho-substituted phenyl h...
[J. Org. Chem. 65(26) , 9268-9271, (2000)] |
|
Studies in Acyl C− H Activation via Aryl and Alkyl to Acyl “...
[Org. Lett. 11(12) , 2591-2593, (2009)] |