A low-barrier hydrogen bond in the catalytic triad of serine proteases.
P A Frey, S A Whitt, J B Tobin
Index: Science 264(5167) , 1927-30, (1994)
Full Text: HTML
Abstract
Spectroscopic properties of chymotrypsin and model compounds indicate that a low-barrier hydrogen bond participates in the mechanism of serine protease action. A low-barrier hydrogen bond between N delta 1 of His57 and the beta-carboxyl group of Asp102 in chymotrypsin can facilitate the formation of the tetrahedral adduct, and the nuclear magnetic resonance properties of this proton indicate that it is a low-barrier hydrogen bond. These conclusions are supported by the chemical shift of this proton, the deuterium isotope effect on the chemical shift, and the properties of hydrogen-bonded model compounds in organic solvents, including the hydrogen bond in cis-urocanic acid, in which the imidazole ring is internally hydrogen-bonded to the carboxyl group.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
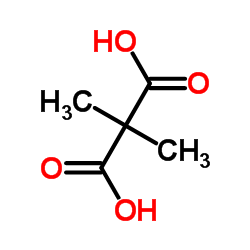 |
2,2-Dimethylmalonic acid
CAS:595-46-0 |
C5H8O4 |
|
Targeted metabolomics in the expanded newborn screening for ...
2015-06-01 [Mol. Biosyst. 11 , 1525-35, (2015)] |
|
Method development in quantitative NMR towards metrologicall...
2015-04-01 [Anal. Bioanal. Chem 407 , 3115-3123, (2015)] |
|
Using high-performance ¹H NMR (HP-qNMR®) for the certificati...
2014-05-01 [J. Pharm. Biomed. Anal. 93 , 102-110, (2014)] |
|
Application of capillary electrophoresis with laser-induced ...
1995-03-01 [Anal. Chem. 67(5) , 812-9, (1995)] |
|
Ion-trap detection of volatile organic compounds in alveolar...
1992-01-01 [Clin. Chem. 38(1) , 60-5, (1992)] |