| Structure | Name/CAS No. | Articles |
|---|---|---|
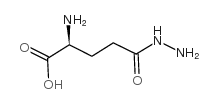 |
L-Glutamic acid,5-hydrazide
CAS:1820-73-1 |
|
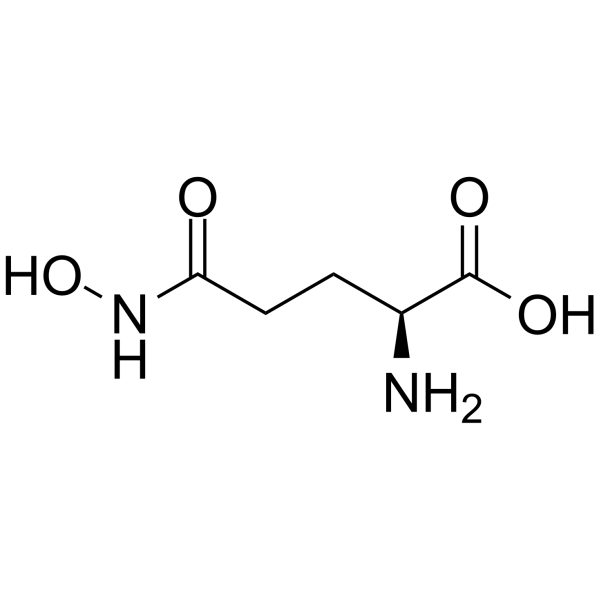 |
L-GlutaMic acid γ-MonohydroxaMate
CAS:1955-67-5 |