Oxazoline-oxazinone oxidative rearrangement. divergent syntheses of (2S,3S)-4,4,4-trifluorovaline and (2S,4S)-5,5,5-Trifluoroleucine.
Julie A Pigza, Tim Quach, Tadeusz F Molinski
Index: J. Org. Chem. 74(15) , 5510-5, (2009)
Full Text: HTML
Abstract
Stereoselective syntheses of the valuable fluorinated amino acids (2S,3S)-4,4,4-trifluorovaline and (2S,4S)-5,5,5-trifluoroleucine have been achieved starting from 4,4,4-trifluoro-3-methylbutanoic acid by using a conceptually simple transformation: conversion to a chiral oxazoline, SeO2-promoted oxidative rearrangement to the dihydro-2H-oxazinone, and face-selective hydrogenation of the C=N bond, followed by hydrogenolysis-hydrolysis. The transformation is limited by the tendency of the intermediate beta-trifluoromethyldihydrooxazinone to undergo imine-enamine isomerization. Both amino acids were obtained as configurationally pure hydrochloride salts identical in all respects with those in literature reports.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
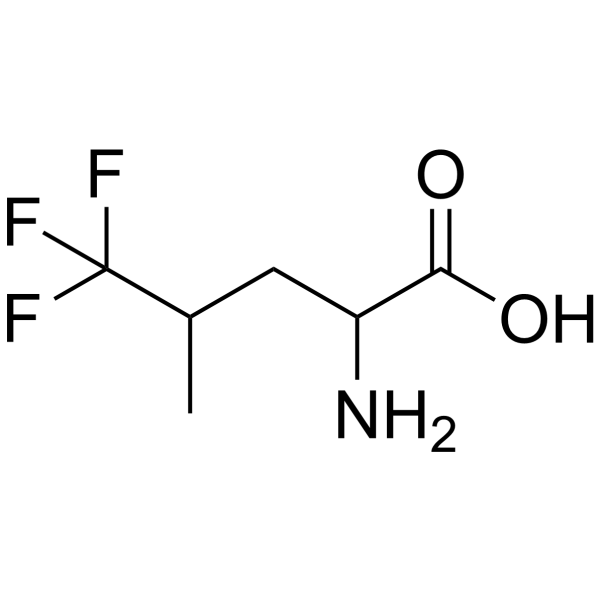 |
5,5,5-TRIFLUORO-DL-LEUCINE
CAS:2792-72-5 |
C6H10F3NO2 |
|
The first isolation of two types of trifluoroleucine resista...
2003-10-01 [Biotechnol. Lett. 25(20) , 1735-8, (2003)] |
|
Construction of two new vectors for transformation of labora...
2004-01-01 [Folia Microbiol. (Praha) 49(5) , 534-8, (2004)] |
|
Self-association and membrane-binding behavior of melittins ...
2001-08-01 [J. Am. Chem. Soc. 123(30) , 7407-13, (2001)] |
|
Biosynthesis and stability of coiled-coil peptides containin...
2009-01-05 [ChemBioChem. 10(1) , 84-6, (2009)] |
|
Stabilization of coiled-coil peptide domains by introduction...
2001-03-06 [Biochemistry 40(9) , 2790-6, (2001)] |