Self-association and membrane-binding behavior of melittins containing trifluoroleucine.
A Niemz, D A Tirrell
Index: J. Am. Chem. Soc. 123(30) , 7407-13, (2001)
Full Text: HTML
Abstract
We have investigated the effect of trifluoroleucine substitution on the membrane-binding and tetramerization behavior of melittin. Analogues were synthesized in which Leu 9, Leu 13, and all four intrinsic leucine residues of melittin were replaced by 5,5,5-trifluoroleucine. Both the mono- and tetra-substituted melittins were found to exhibit stronger self-association and enhanced affinity for lipid bilayer membranes, compared to the wild-type peptide. The extent of the observed effects depends on the site of introduction of trifluoroleucine and, in the case of substitution at position 13, on the stereochemistry of the trifluoroleucine side chain. Analysis of the membrane association isotherms is consistent with aggregation of fluorinated melittins within the lipid bilayer. These results suggest that fluorocarbon-hydrocarbon separation, in addition to an increase in hydrophobic character, contributes to enhanced membrane binding.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
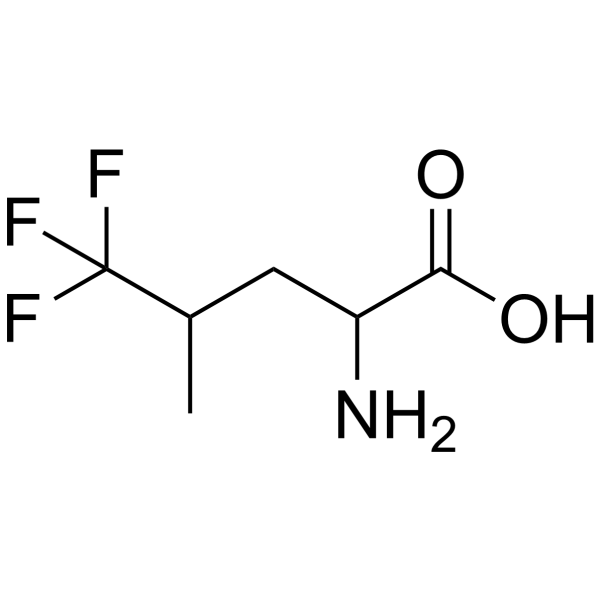 |
5,5,5-TRIFLUORO-DL-LEUCINE
CAS:2792-72-5 |
C6H10F3NO2 |
|
The first isolation of two types of trifluoroleucine resista...
2003-10-01 [Biotechnol. Lett. 25(20) , 1735-8, (2003)] |
|
Construction of two new vectors for transformation of labora...
2004-01-01 [Folia Microbiol. (Praha) 49(5) , 534-8, (2004)] |
|
Biosynthesis and stability of coiled-coil peptides containin...
2009-01-05 [ChemBioChem. 10(1) , 84-6, (2009)] |
|
Stabilization of coiled-coil peptide domains by introduction...
2001-03-06 [Biochemistry 40(9) , 2790-6, (2001)] |
|
Oxazoline-oxazinone oxidative rearrangement. divergent synth...
2009-08-07 [J. Org. Chem. 74(15) , 5510-5, (2009)] |