Reactions of morphine derivatives with phenyliodo(III)diacetate (PIDA): synthesis of new morphine analogues.
S Garadnay, P Herczegh, S Makleit, T Friedmann, P Riba, S Fürst
Index: Curr. Med. Chem. 8(6) , 621-6, (2001)
Full Text: HTML
Abstract
The reactions of morphine and its derivatives with phenyliodo(III)diacetate (PIDA) have been studied. This methodology has not been introduced to morphine alkaloids, despite the fact that such a strategy would ensure dearomatization of the electrophilic aromatic ring of morphine derivatives leading to nucleophilic ortho-quinoidal structures with potential pharmacological interest. The products, formed in regio- and diastereoselective or diastereospecific reactions, carry mixed-acetal or 1,3-dioxolane moieties. At low concentrations 6a has mu-opioid agonist character but in higher concentrations showed a non receptorial antagonist effect on isolated mouse vas deferens.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
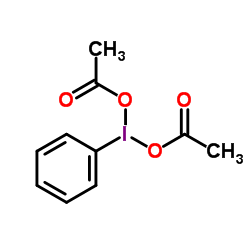 |
(Diacetoxyiodo)benzene
CAS:3240-34-4 |
C10H11IO4 |
|
An expedient procedure for the oxidative cleavage of olefini...
2010-04-02 [Org. Lett. 12(7) , 1552-5, (2010)] |
|
Palladium-catalyzed oxygenation of unactivated sp3 C-H bonds...
2004-08-11 [J. Am. Chem. Soc. 126 , 9542-9543, (2004)] |
|
Determination of vitamin C in tablets and fruits by titratio...
1982-01-01 [Il Farmaco 37(1) , 9-14, (1982)] |
|
Copper-catalyzed diacetoxylation of olefins using PhI(OAc)2 ...
2010-04-02 [Org. Lett. 12(7) , 1412-5, (2010)] |
|
Synthesis of benzimidazoles by PIDA-promoted direct C(sp2)-H...
2012-10-29 [Chemistry 18(44) , 13964-7, (2012)] |