| Structure | Name/CAS No. | Articles |
|---|---|---|
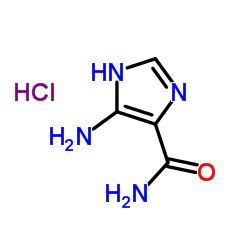 |
5-Amino-1H-imidazole-4-carboxamide hydrochloride
CAS:72-40-2 |
|
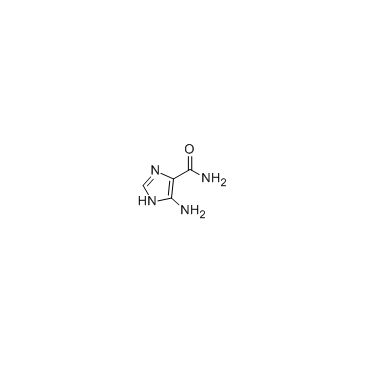 |
5-Amino-4-imidazolecarboxamide
CAS:360-97-4 |
|
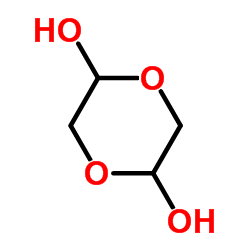 |
1,4-Dioxane-2,5-diol
CAS:23147-58-2 |