| Structure | Name/CAS No. | Articles |
|---|---|---|
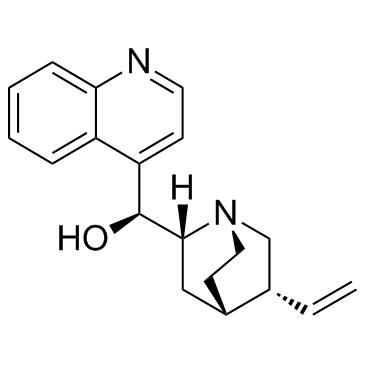 |
Cinchonine
CAS:118-10-5 |
|
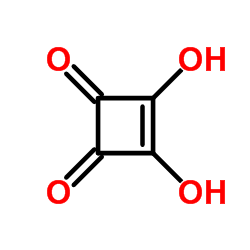 |
Squaric acid
CAS:2892-51-5 |
|
 |
(9S)-Cinchonan-9-ol sulfate (2:1)
CAS:5949-16-6 |