| Structure | Name/CAS No. | Articles |
|---|---|---|
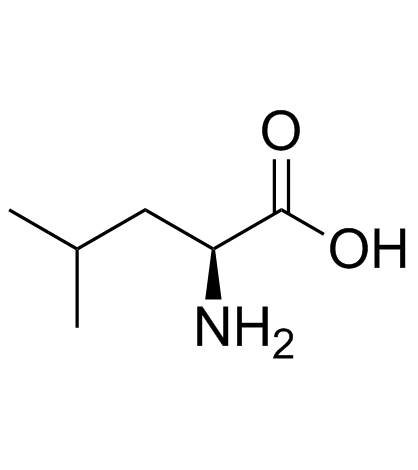 |
L-leucine
CAS:61-90-5 |
|
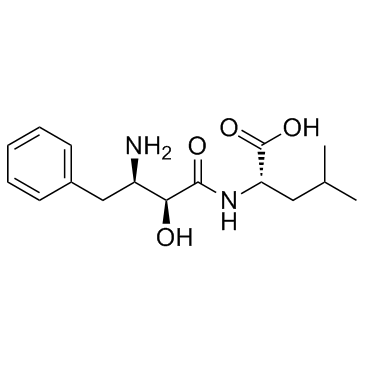 |
Ubenimex
CAS:58970-76-6 |
|
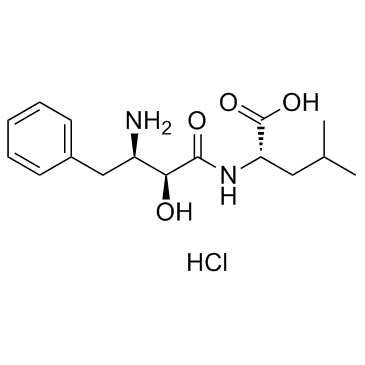 |
Bestatin hydrochloride
CAS:65391-42-6 |