| Structure | Name/CAS No. | Articles |
|---|---|---|
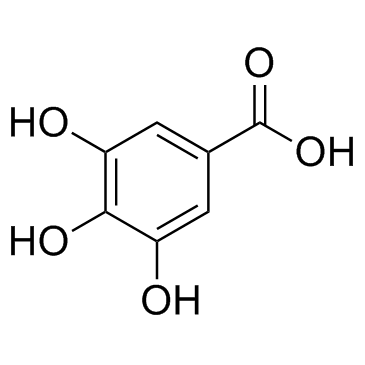 |
Gallic acid
CAS:149-91-7 |
|
 |
Leonurine
CAS:24697-74-3 |
|
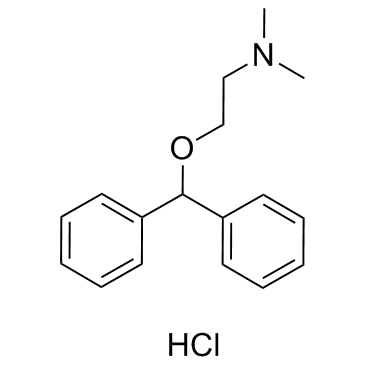 |
Diphenhydramine Hydrochloride
CAS:147-24-0 |