| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Aspirin
CAS:50-78-2 |
|
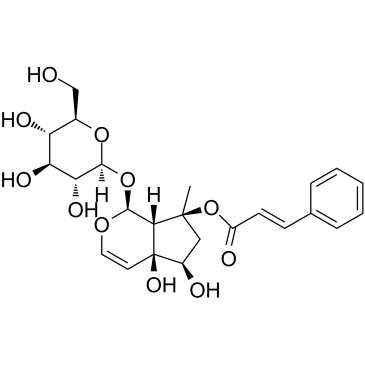 |
Harpagoside
CAS:19210-12-9 |
|
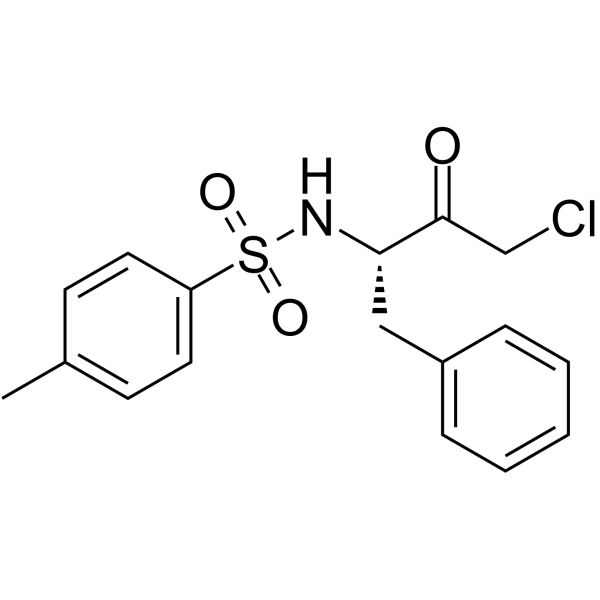 |
Tosyl phenylalanyl chloromethyl ketone
CAS:402-71-1 |
|
 |
Dexamethasone
CAS:50-02-2 |