Crystal structure of a heterodimeric complex of RAR and RXR ligand-binding domains.
W Bourguet, V Vivat, J M Wurtz, P Chambon, H Gronemeyer, D Moras
Index: Mol. Cell. 5(2) , 289-98, (2000)
Full Text: HTML
Abstract
The crystal structure of a heterodimer between the ligand-binding domains (LBDs) of the human RARalpha bound to a selective antagonist and the constitutively active mouse RXRalphaF318A mutant shows that, pushed by a bulky extension of the ligand, RARalpha helix H12 adopts an antagonist position. The unexpected presence of a fatty acid in the ligand-binding pocket of RXRalpha(F318A is likely to account for its apparent "constitutivity." Specific conformational changes suggest the structural basis of pure and partial antagonism. The RAR-RXR heterodimer interface is similar to that observed in most nuclear receptor (NR) homodimers. A correlative analysis of 3D structures and sequences provides a novel view on dimerization among members of the nuclear receptor superfamily.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
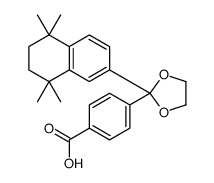 |
SR 11237
CAS:146670-40-8 |
C24H28O4 |
|
Retinoids Bias Integrin Expression and Function in Cutaneous...
2015-08-01 [J. Invest. Dermatol. 135 , 2102-8, (2015)] |
|
Pharmacological evaluation of the mechanisms involved in inc...
2016-03-01 [Toxicol. Appl. Pharmacol. 294 , 32-42, (2016)] |
|
Detection of a receptor-ligand non-covalent complex using a ...
2002-01-01 [Rapid Commun. Mass Spectrom. 16(20) , 2003-6, (2002)] |
|
The "Phantom Effect" of the Rexinoid LG100754: structural an...
2010-01-01 [PLoS ONE 5(11) , e15119, (2010)] |
|
Retinoid X receptor-selective ligands produce malformations ...
1996-03-05 [Proc. Natl. Acad. Sci. U. S. A. 93(5) , 1803-7, (1996)] |