| Structure | Name/CAS No. | Articles |
|---|---|---|
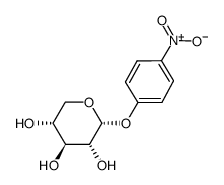 |
P-NITROPHENYL α-D-XYLOPYRANOSIDE
CAS:10238-28-5 |
|
 |
4-Nitrophenyl-beta-D-xylopyranoside
CAS:2001-96-9 |
|
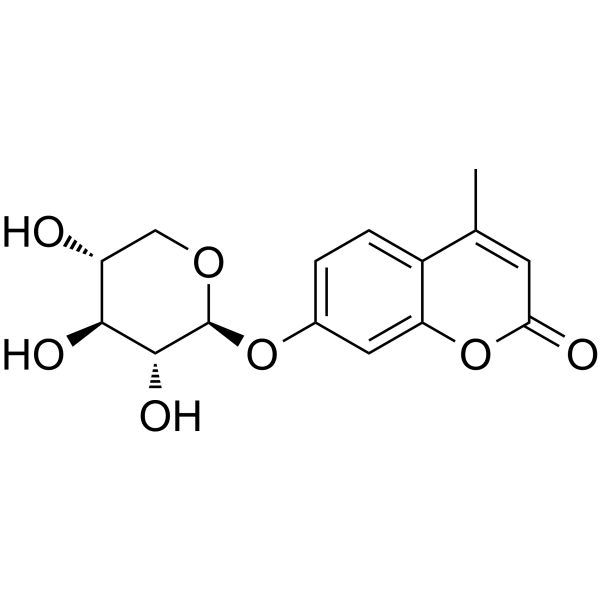 |
(4-Methylumbelliferone)-β-D-xylopyranoside
CAS:6734-33-4 |