Solvent effect on the charge transfer complex of oxatomide with 2,3-dichloro-5,6-dicyanobenzoquinone.
M Pandeeswaran, K P Elango
Index: Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 65(5) , 1148-53, (2006)
Full Text: HTML
Abstract
The charge transfer complex (CT-complex) between oxatomide drug and 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) was studied spectrophotometrically in 10 solvents at different temperatures. The donor oxatomide is found to form stable 1:1 stoichiometric complex with DDQ and the stoichiometry was unaffected by change in polarity of the solvent studied. The DeltaH degrees, DeltaS degrees and DeltaG degrees values are all negative, so the studied complex is reasonably stable and exothermic in nature. The ionization potential of the drug was determined using the CT-absorption bands of the complex in all the solvents. The dissociation energy of the charge transfer excited state for the CT-complex in different solvents was also determined and is found to be constant. The spectroscopic and thermodynamic properties were observed to be sensitive to the nature of the solvent.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
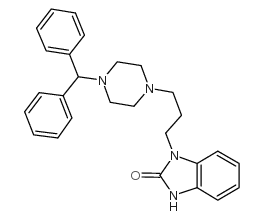 |
Oxatomide
CAS:60607-34-3 |
C27H30N4O |
|
Treatment of allergic rhinitis in infants and children: effi...
2009-01-01 [Drugs 69(18) , 2541-76, (2009)] |
|
Differential thermodynamic driving force of first- and secon...
2014-09-15 [Biochem. Pharmacol. 91(2) , 231-41, (2014)] |
|
Molecular complexes of ketaconazole and oxatomide with p-chl...
2011-09-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 79(5) , 1137-44, (2011)] |
|
High-performance liquid chromatographic determination of oxa...
2002-01-01 [Arzneimittelforschung 52(10) , 754-63, (2002)] |
|
Effect of oxatomide, an antiallergic agent, on QT interval i...
2001-01-01 [Arzneimittelforschung 51(12) , 971-6, (2001)] |