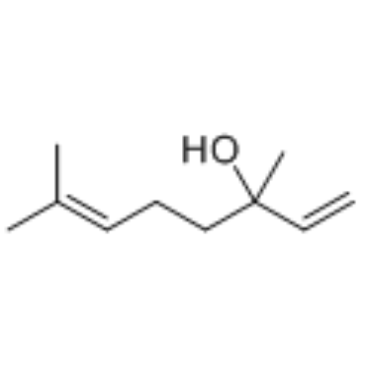
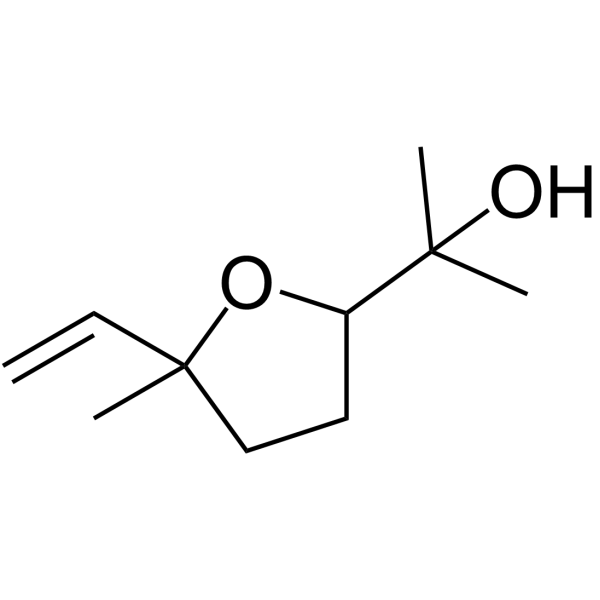
|
~78% |
|
~0% |
|
~96% |
|
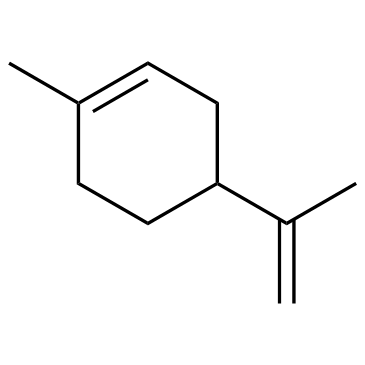
~83% |
|
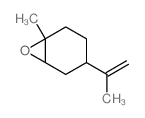
~0% |
![1-methyl-4-(2-methyloxiranyl)-7-oxabicyclo[4.1.0]heptane Structure](https://image.chemsrc.com/caspic/093/96-08-2.png)
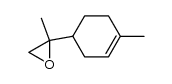
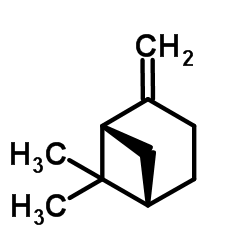
![[1R-(1alpha,2alpha,5alpha)]-6,6-dimethylspiro[bicyclo[3.1.1]heptane-2,2'-oxirane] Structure](https://image.chemsrc.com/caspic/403/56246-58-3.png)
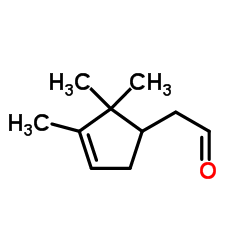
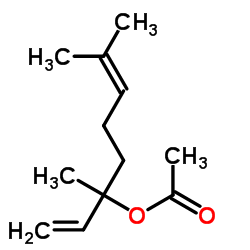
![[5-(3,3-dimethyloxiran-2-yl)-3-methylpent-1-en-3-yl] acetate Structure](https://image.chemsrc.com/caspic/391/41610-76-8.png)