Steroids
1990-11-01
A new efficient synthetic method for 2- and 4-hydroxy-17 alpha-ethynylestradiol.
R G Xie, Q Y Chen, J Xie, H M Zhao
Index: Steroids 55(11) , 488-90, (1990)
Full Text: HTML
Abstract
The reaction of ethyl magnesium bromide and 17 alpha-ethynylestradiol with formaldehyde in the presence of triethyl phosphate or hexamethylphosphoramide gave the 2- and 4-formyl-17 alpha-ethynylestradiol in high yield. Treatment of the formyl derivatives with an alkaline solution of hydrogen peroxide in tetrahydrofuran afforded the corresponding catechols in almost quantitative yield. This new synthetic method was far superior to other methods, especially concerning simplicity, selectivity, and high yields.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
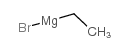 |
ETHYLMAGNESIUM BROMIDE
CAS:925-90-6 |
C2H5BrMg |
Related Articles:
More...
|
New reagent system for attaining high regio- and stereoselec...
2005-01-20 [Org. Lett. 7(2) , 183-6, (2005)] |
|
Rhodococcus opacus strain PD630 as a new source of high-valu...
2000-05-01 [Microbiology 146 ( Pt 5) , 1143-9, (2000)] |
|
Studies of the formation of all-carbon quaternary centres, e...
2004-05-21 [Org. Biomol. Chem. 2(10) , 1447-55, (2004)] |
|
A direct method for regiospecific analysis of TAG using alph...
2002-08-01 [Lipids 37(8) , 817-21, (2002)] |
|
Preparation of ethyl magnesium bromide for regiospecific ana...
2008-01-01 [J. Oleo Sci. 57(8) , 459-62, (2008)] |