Enzymatic synthesis of 4-amino-3,5-diethylphenyl sulfate, a rodent metabolite of alachlor.
S J Logusch, P C Feng, H Fujiwara, W C Hutton, S J Wratten
Index: J. Agric. Food Chem. 47(5) , 2125-9, (1999)
Full Text: HTML
Abstract
Rat liver tissue homogenates were utilized for in vitro enzymatic conversion of 2,6-diethylaniline (DEA) to the important alachlor metabolite 4-amino-3,5-diethylphenyl sulfate (ADEPS), which was also generated as a radiolabeled standard for use in metabolism studies. ADEPS formation in rodents is associated with the production of other reactive metabolites implicated in alachlor rodent carcinogenesis, making dependable access to an ADEPS standard highly desirable. (14)C-DEA was oxidized by rat liver microsomes to (14)C-4-amino-3,5-diethylphenol, which was further converted to ADEPS via addition of the phosphosulfate transferase cofactor adenosine-3'-phosphate-5'-phosphosulfate. Microprobe NMR was used in conjunction with high-resolution mass spectrometry to fully characterize the resulting (14)C-ADEPS and confirm its structure. Because microgram quantities sufficed for full characterization, the enzymatic transformation provides a viable alternative to radiosynthesis of (14)C-ADEPS.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
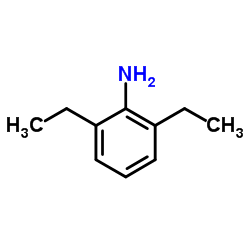 |
2,6-Diethylaniline
CAS:579-66-8 |
C10H15N |
|
Ferrocene-based guanidine derivatives: in vitro antimicrobia...
2014-10-06 [Eur. J. Med. Chem. 85 , 438-49, (2014)] |
|
Further evidence of an inverted region in proton transfer wi...
2006-05-25 [J. Phys. Chem. A 110(20) , 6408-14, (2006)] |
|
Spectroscopic studies of molecular interactions involving 2,...
2007-01-01 [Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 66(1) , 94-101, (2007)] |
|
Biodegradation of alachlor by soil streptomycetes.
2004-06-01 [Appl. Microbiol. Biotechnol. 64(5) , 712-7, (2004)] |
|
Determination of alachlor and its metabolite 2,6-diethylanil...
2011-08-10 [J. Agric. Food Chem. 59(15) , 8078-85, (2011)] |