| Structure | Name/CAS No. | Articles |
|---|---|---|
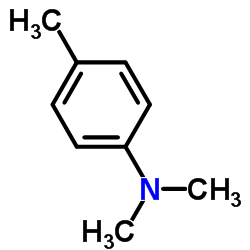 |
4,N,N-Trimethylaniline
CAS:99-97-8 |
|
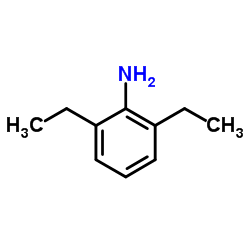 |
2,6-Diethylaniline
CAS:579-66-8 |