S(+)methylenedioxy-N-n-propylnoraporphine: an orally active inhibitor of dopamine selective for rat limbic system.
A Campbell, R J Baldessarini, N S Kula, V J Ram, J L Neumeyer
Index: Brain Res. 403(2) , 393-7, (1987)
Full Text: HTML
Abstract
The 10-11-methylenedioxy (MDO) derivative of S(+)N-n-propylnorapomorphine (NPA) was prepared and tested as a possible active prodrug to S(+)NPA, which we have recently found to exert in vivo activity suggestive of selective antagonism of dopamine receptors in the limbic forebrain but not the extrapyramidal basal ganglia. Like S(+)NPA, S(+)MDO-NPA inhibited the behavioral arousal induced by dopamine injected into nucleus accumbens of the rat, but not the head-turning response to dopamine injected into the corpus striatum. However, only MDO-NPA was orally active and it was somewhat longer-acting than NPA. The activity of S(+)MDO-NPA was prevented by pretreatment with the oxidase inhibitor SKF-525A. These properties are analogous to those of R(-)MDO-NPA, which we had previously reported as an orally active prodrug of the dopamine agonist R(-)NPA. Thus the methylenedioxy derivatives of the two entantiomers of NPA have properties desirable in a potentially clinically useful dopamine agonist and limbic dopamine antagonist, respectively.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
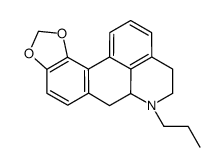 |
(-)-MDO-NPA HCl
CAS:81264-57-5 |
C20H21NO2 |
|
Behavioral effects of (-)10,11-methylenedioxy-N-n-propylnora...
1982-10-01 [Neuropharmacology 21(10) , 953-61, (1982)] |
|
Dopaminergic stimulation of oxytocin concentrations in the p...
1992-09-01 [J. Clin. Endocrinol. Metab. 75(3) , 855-60, (1992)] |
|
Tissue levels of N-n-propylnorapomorphine after treatment wi...
1982-12-01 [Neuropharmacology 21(12) , 1311-6, (1982)] |
|
An orally effective, long-acting dopaminergic prodrug: (-)-1...
1982-01-08 [Eur. J. Pharmacol. 77(1) , 87-8, (1982)] |
|
(-)-10,11-Methylenedioxy-N-propylnoraporphine, an orally eff...
1983-03-25 [Eur. J. Pharmacol. 88(2-3) , 273-4, (1983)] |