| Structure | Name/CAS No. | Articles |
|---|---|---|
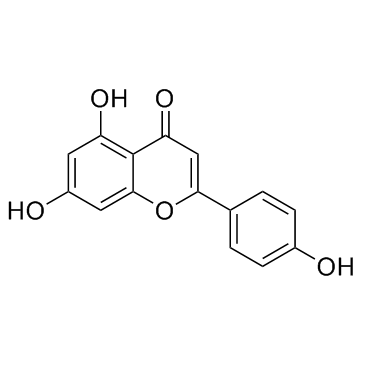 |
Apigenin
CAS:520-36-5 |
|
 |
Resveratrol
CAS:501-36-0 |
|
 |
Genistein
CAS:446-72-0 |
|
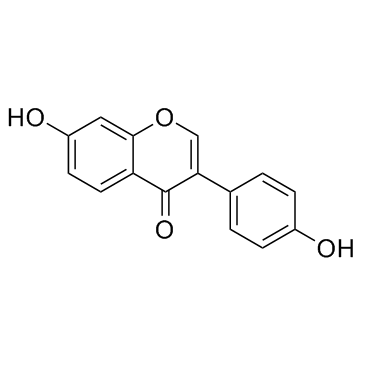 |
Daidzein
CAS:486-66-8 |
|
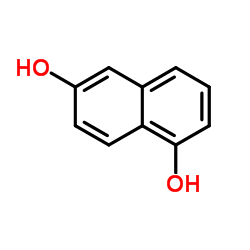 |
1,6-Dihydroxynaphthalene
CAS:575-44-0 |
|
 |
2,7-Dihydroxynaphthalene
CAS:582-17-2 |