The chiral separation of triazole pesticides enantiomers by amylose-tris (3,5-dimethylphenylcarbamate) chiral stationary phase.
Peng Wang, Donghui Liu, Shuren Jiang, Yangguang Xu, Zhiqiang Zhou
Index: J. Chromatogr. Sci. 46(9) , 787-92, (2008)
Full Text: HTML
Abstract
The amylose-tris(3,5-dimethylphenylcarbamate) chiral stationary phase was synthesized and used to separate the enantiomers of triazole pesticides by high-performance liquid chromatography. The mobile phase was n-hexane-isopropanol applying a flow rate of 1.0 mL/min. Six triazole pesticides were enantioselectively separated. Myclobutanil, paclobutrazol, tebuconazole, and uniconazole obtained complete separation with the resolution factors of 5.73, 2.99, 1.72, and 2.07, respectively, and imazalil and diniconazole obtained partial separation with the resolution factors of 0.79 and 0.77 under the optimized conditions. The effect of the content of isopropanol as well as column temperature on the separation was investigated. A circular dichroism detector was used to identify the enantiomers and determine the elution orders. The results showed the low temperature was good for the chiral separation except for diniconazole. The thermodynamic parameters calculated based on linear Van't Hoff plots showed the chiral separations were controlled by enthalpy.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
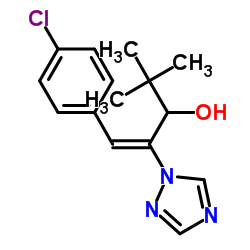 |
Prunit
CAS:83657-22-1 |
C15H18ClN3O |
|
Improved biological effects of uniconazole using porous holl...
2012-03-01 [Pest Manag. Sci. 68(3) , 437-43, (2012)] |
|
Formation of embryogenic cell clumps from carrot epidermal c...
2005-01-01 [J. Plant Physiol. 162(1) , 47-54, (2005)] |
|
Enantiomeric resolution and growth-retardant activity in ric...
2012-01-11 [J. Agric. Food Chem. 60(1) , 160-4, (2012)] |
|
Involvement of gibberellin in tracheary element differentiat...
2006-09-01 [Protoplasma 228(4) , 179-87, (2006)] |
|
Plants with increased expression of ent-kaurene oxidase are ...
2005-02-01 [Plant Cell Physiol. 46(2) , 284-91, (2005)] |