An automated, highly sensitive LC-MS/MS assay for the quantification of the opiate antagonist naltrexone and its major metabolite 6beta-naltrexol in dog and human plasma.
Claudia Clavijo, Jamie Bendrick-Peart, Yan Ling Zhang, Gillian Johnson, Antje Gasparic, Uwe Christians
Index: J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 874(1-2) , 33-41, (2008)
Full Text: HTML
Abstract
To support animal studies and clinical pharmacokinetic trials, we developed and validated an automated, specific and highly sensitive LC-MS/MS method for the quantification of naltrexone and 6beta-naltrexol in the same run. In human plasma, the assay had a lower limit of quantitation of only 5pg/mL. This was of critical importance to follow naltrexone pharmacokinetics during its terminal elimination phase. The assay had the following key performance characteristics for naltrexone in human plasma: range of reliable quantification: 0.005-100ng/mL (r2>0.99), inter-day accuracy (0.03ng/mL): 103.7% and inter-day precision: 10.1%. There were no ion suppression, matrix interferences or carry-over.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
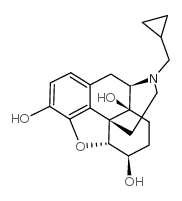 |
6β-Naltrexol
CAS:49625-89-0 |
C20H25NO4 |
|
Conformational analysis of 6α- and 6β-naltrexol and derivati...
2012-02-27 [J. Chem. Inf. Model. 52(2) , 391-5, (2012)] |
|
In vivo evaluation of a transdermal codrug of 6-beta-naltrex...
2008-04-23 [Eur. J. Pharm. Sci. 33(4-5) , 371-9, (2008)] |
|
Comparison of naltrexone, 6alpha-naltrexol, and 6beta-naltre...
2008-01-01 [Psychopharmacology 195(4) , 479-86, (2008)] |
|
Comparison of the opioid receptor antagonist properties of n...
2008-03-31 [Eur. J. Pharmacol. 583(1) , 48-55, (2008)] |
|
Determination of naltrexone and 6beta-naltrexol in human blo...
2010-02-01 [Anal. Bioanal. Chem 396(3) , 1249-57, (2010)] |