Cytotoxicity of semisynthetic acetal triterpenes from one-pot vicinal diol cleavage following by lactolization: reaction promoted by NaIO4/SiO2 gel in THF.
Louis Pergaud Sandjo, Aurelie Vigee Barry Songfack Djoumessi, Vincent Rincheval, Hervé Martial Poumale Poumale, Berhanu M Abegaz, Bonaventure T Ngadjui
Index: Nat. Prod. Res. 27(8) , 711-8, (2013)
Full Text: HTML
Abstract
In situ C-C bond cleavage of vicinal diol following by the lactolisation resulted from separated treatment of Arjunolic acid (1), 24-hydroxytormentic acid (2) and 3-O-β-D-glucopyranosylsitosterol (3) with sodium periodate and silica gel in dried THF according to the strategic position of hydroxyl functions in the molecule. The reaction led to a lactol pentacyclic triterpenes 1A, 2A and a bicyclotriacetal of β-sitosterol 3A. These products were further acetylated and the cytotoxicity of all molecules was evaluated against human fibrosarcoma HT1080 cancer cells lines.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
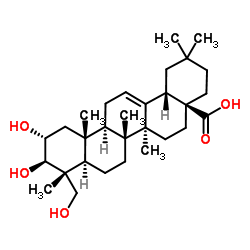 |
Arjunolic acid
CAS:465-00-9 |
C30H48O5 |
|
Chemical constituents of Tilia taquetii leaves and their inh...
2014-12-01 [Nat. Prod. Commun. 9(12) , 1683-5, (2014)] |
|
[Triterpenoids from leaves of Ilex cornuta].
2009-04-01 [Zhongguo Zhong Yao Za Zhi 34(8) , 999-1001, (2009)] |
|
Disruption of glucose tolerance caused by glucocorticoid exc...
2014-10-01 [Indian J. Exp. Biol. 52(10) , 972-82, (2014)] |
|
Prophylactic role of arjunolic acid in response to streptozo...
2009-10-30 [Chem. Biol. Interact. 181(3) , 297-308, (2009)] |
|
Triterpenoid saponins from Symplocos lancifolia.
2011-02-25 [J. Nat. Prod. 74 , 163-8, (2011)] |