| Structure | Name/CAS No. | Articles |
|---|---|---|
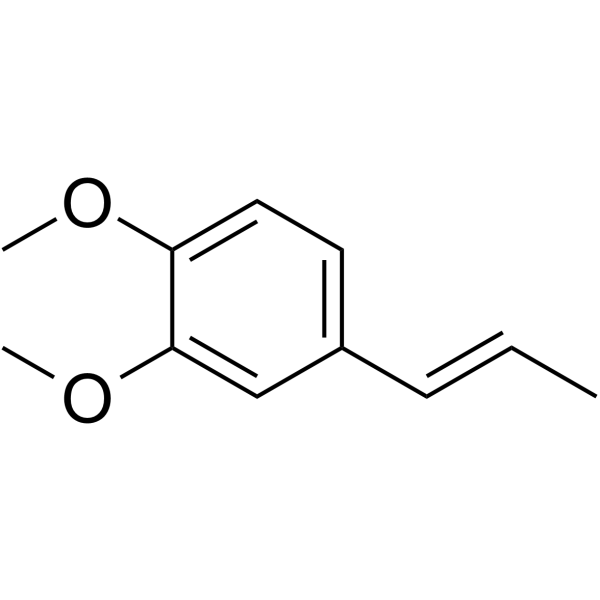 |
(E)-methyl isoeugenol
CAS:93-16-3 |
|
 |
trans-4-phenylbut-3-en-2-one
CAS:1896-62-4 |
|
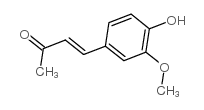 |
4-(4-hydroxy-3-methoxyphenyl)-3-buten-2-one
CAS:1080-12-2 |
|
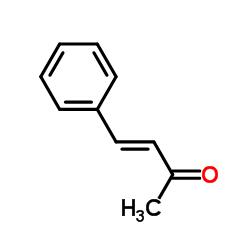 |
Benzalacetone
CAS:122-57-6 |