Determination of isoniazid, acetylisoniazid, acetylhydrazine and diacetylhydrazine in biological fluids by high-performance liquid chromatography.
W von Sassen, M Castro-Parra, E Musch, M Eichelbaum
Index: J. Chromatogr. A. 338(1) , 113-22, (1985)
Full Text: HTML
Abstract
A high-performance liquid chromatographic assay for the determination of isoniazid, acetylisoniazid, acetylhydrazine and diacetylhydrazine (plasma and urine) was developed. The m-chlorobenzoyl derivatives of isoniazid, acetylhydrazine and the internal standard propionylhydrazine were prepared, separated on a RP-18 column and detected at 220 nm. Acetylisoniazid, diacetylhydrazine and the internal standard dipropionylhydrazine were converted to isoniazid, acetylhydrazine, and propionylhydrazine by acidic hydrolysis and subsequently derivatized with m-fluorobenzoyl chloride, separated on a RP-18 column and detected at 220 nm. The lower limits of detection in plasma are acetylhydrazine 0.5 nmol/ml, isoniazid 1.0 nmol/ml, diacetylhydrazine 1.0 nmol/ml and acetylisoniazid 2.0 nmol/ml, and in urine, acetylhydrazine 10 nmol/ml, isoniazid 15 nmol/ml, diacetylhydrazine 20 nmol/ml and acetylisoniazid 40 nmol/ml. This method is sensitive, reproducible, accurate and precise; therefore, it is well suited for detailed pharmacokinetic studies.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
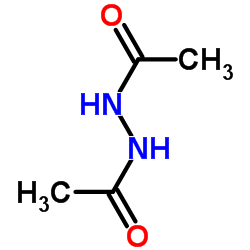 |
N'-Acetylacetohydrazide
CAS:3148-73-0 |
C4H8N2O2 |
|
A charged aerosol detector/chemiluminescent nitrogen detecto...
2015-09-11 [J. Chromatogr. A. 1411 , 63-8, (2015)] |
|
Urinary metabolic profile of isoniazid in patients who devel...
1984-12-01 [Hum. Toxicol. 3(6) , 485-95, (1984)] |
|
Levels of acetyl hydrazines and rate of acetylation of isoni...
1985-01-01 [Indian J. Physiol. Pharmacol. 29(2) , 83-8, (1985)] |
|
Effect of isoniazid on folic acid status in Swiss mice and r...
1985-01-01 [Indian J. Physiol. Pharmacol. 29(3) , 133-8, (1985)] |
|
Double N-H bond activation of N,N'-bis-substituted hydrazine...
2012-02-07 [Dalton Trans. 41(5) , 1529-33, (2012)] |