Double N-H bond activation of N,N'-bis-substituted hydrazines with stable N-heterocyclic silylene.
Ramachandran Azhakar, Rajendra S Ghadwal, Herbert W Roesky, Jakob Hey, Dietmar Stalke
Index: Dalton Trans. 41(5) , 1529-33, (2012)
Full Text: HTML
Abstract
The reaction of N-heterocyclic silylene (NHSi) L [L = CH{(C[double bond, length as m-dash]CH(2))(CMe)(2,6-iPr(2)C(6)H(3)N)(2)}Si] with benzoylhydrazine, 1,2-dicarbethoxyhydrazine, 1,2-diacetylhydrazine and 1,2-bis(tert-butoxycarbonyl)hydrazine in 1 : 1 molar ratio resulted in compounds 1-4 with an almost quantitative yield and five coordinate silicon atoms. Compounds 1-4 were formed by double N-H bond activation by deliberate selection of N,N'-bis-substituted hydrazine compounds bearing the -C(O)NHNH- unit. Compounds 1-4 were characterized by NMR spectroscopy, EI-MS and elemental analysis. The molecular structures of compounds 1-3 were unambiguously established by single crystal X-ray structural analysis.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
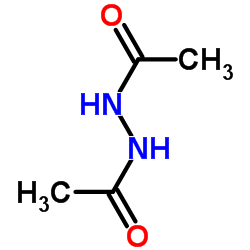 |
N'-Acetylacetohydrazide
CAS:3148-73-0 |
C4H8N2O2 |
|
A charged aerosol detector/chemiluminescent nitrogen detecto...
2015-09-11 [J. Chromatogr. A. 1411 , 63-8, (2015)] |
|
Urinary metabolic profile of isoniazid in patients who devel...
1984-12-01 [Hum. Toxicol. 3(6) , 485-95, (1984)] |
|
Levels of acetyl hydrazines and rate of acetylation of isoni...
1985-01-01 [Indian J. Physiol. Pharmacol. 29(2) , 83-8, (1985)] |
|
Effect of isoniazid on folic acid status in Swiss mice and r...
1985-01-01 [Indian J. Physiol. Pharmacol. 29(3) , 133-8, (1985)] |
|
Pharmacokinetics of the toxic hydrazino metabolites formed f...
1985-12-01 [J. Pharmacol. Exp. Ther. 235(3) , 566-70, (1985)] |