| Structure | Name/CAS No. | Articles |
|---|---|---|
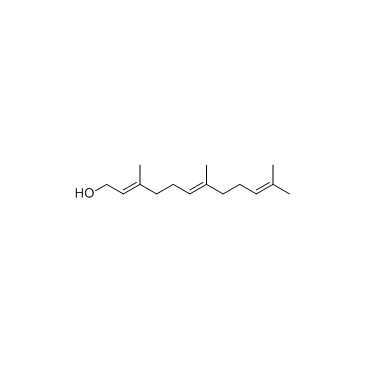 |
farnesol
CAS:4602-84-0 |
|
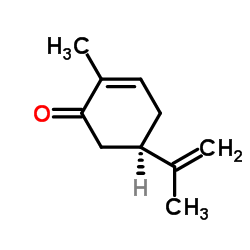 |
L(-)-Carvone
CAS:6485-40-1 |
|
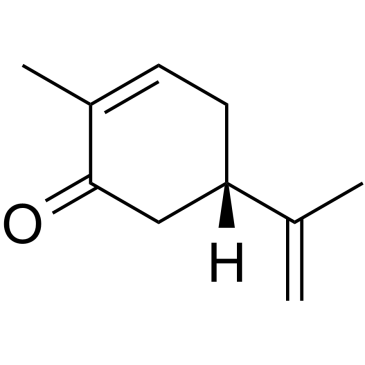 |
(S)-2-Methyl-5-(prop-1-en-2-yl)cyclohex-2-enone
CAS:2244-16-8 |
|
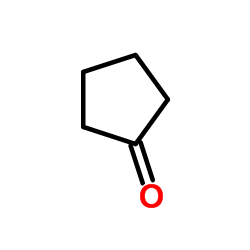 |
Cyclopentanone
CAS:120-92-3 |