| Structure | Name/CAS No. | Articles |
|---|---|---|
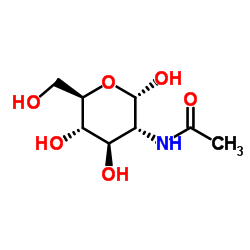 |
methyl 2-acetamido-2-deoxy-alpha-d-glucopyranoside
CAS:6082-04-8 |
|
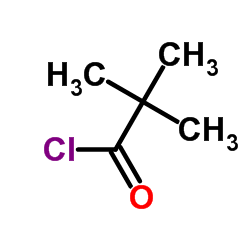 |
Pivaloyl chloride
CAS:3282-30-2 |