| Structure | Name/CAS No. | Articles |
|---|---|---|
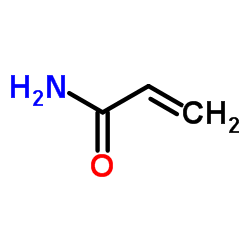 |
Acrylamide Crystals
CAS:79-06-1 |
|
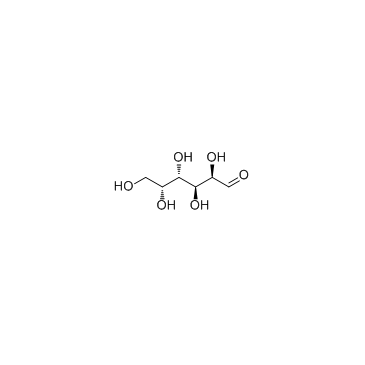 |
D-Galactose
CAS:59-23-4 |
|
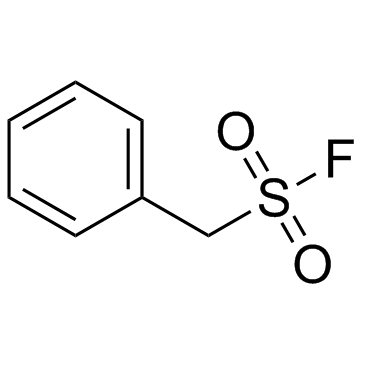 |
PMSF
CAS:329-98-6 |
|
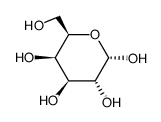 |
galactose
CAS:3646-73-9 |
|
 |
N-Succinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate
CAS:64987-85-5 |